stability test chamber.jpg)
Enquiry
- Applications
- Features
- Control
- Construction
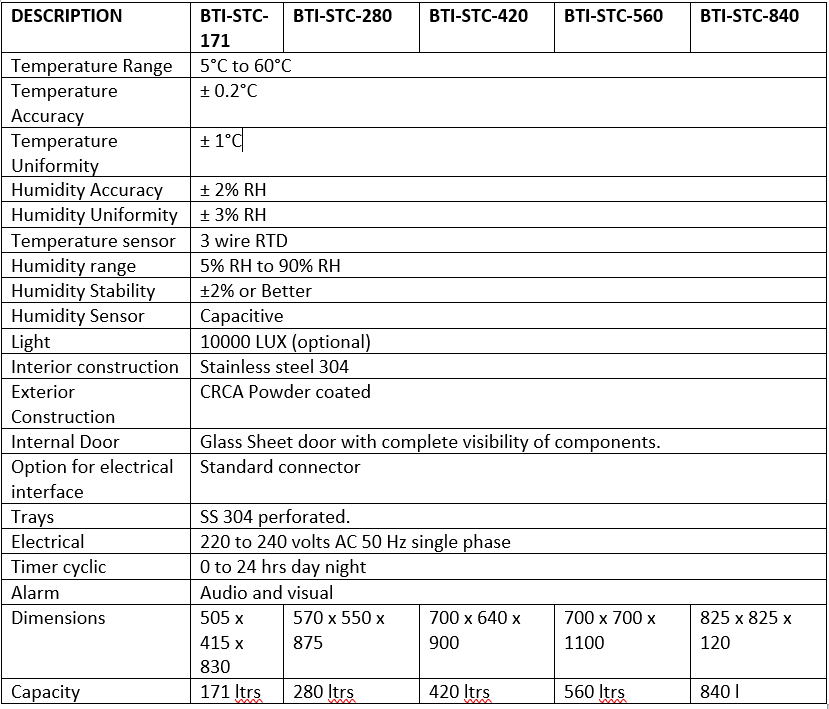
- Specification
BioGene Stability Chambers are a manufacturer and exporter of humidity chambers and stability chambers that are used for stability testing under ICH guidelines. The chambers are designed to maintain a stable temperature and humidity environment, which is essential for testing the shelf life of drugs and other pharmaceutical products.
Bio-Gene Temperature and Humidity Chamber have:
- A wide temperature range of 5°C to 60°C
- A capacity of 150 liters to 1000 liters
- 21 CFR part 11 software for compliance with regulatory requirements
- An intuitive user interface
- Low Water Level Alarm System
- A sturdy and durable construction
- Meets photostability requirements for aerospace, defense, pharma, medical, and automobile industries
- Standards : ICH-Q1AR2
- ISO,EN, CE, Electro magnetic
- MDD :- 93/42/EEC
- IEC 60068-2-38
- EN 61000-6-1 : 2001
- Electromagnetic Compatibility Directive (89)/336/EEC)
- EN: 60101, ISO, CE
- Touch Screen Type 5.7’/ 7.0”
- Double walled with PUF insulation of 80-150 mm
- Inner Chamber: Stainless Steel 304
- Outer Chamber: Mild Steel Powder Coated
- Danfoss Tecumseh CFC free cooling system with hermetically sealed compressor with temperature sensors
- Stainless steel Trays
- Inner Door: Full-length inner glass door
- WiFi & LAN based data Communication
- Fluorescent light
- Forced air circulation for uniform temperature
- Safety devices: Low & high temperature cut-off settable thermostats & high humidity cut-off device
- Audio/Visual alarms for deviations
- DATA LOGGING- data Logging up logs for Temp.& Humidity
RS232 Data Logger


HUMIDITY-TEST-CHAMBER.jpg)
climate TEST chamber.jpg)
REACH IN ALTITUDE TEST CHAMBER.jpg)
XENON-LAMP-WEATHERING-TE.jpg)

battery-test-chamber.jpg)


