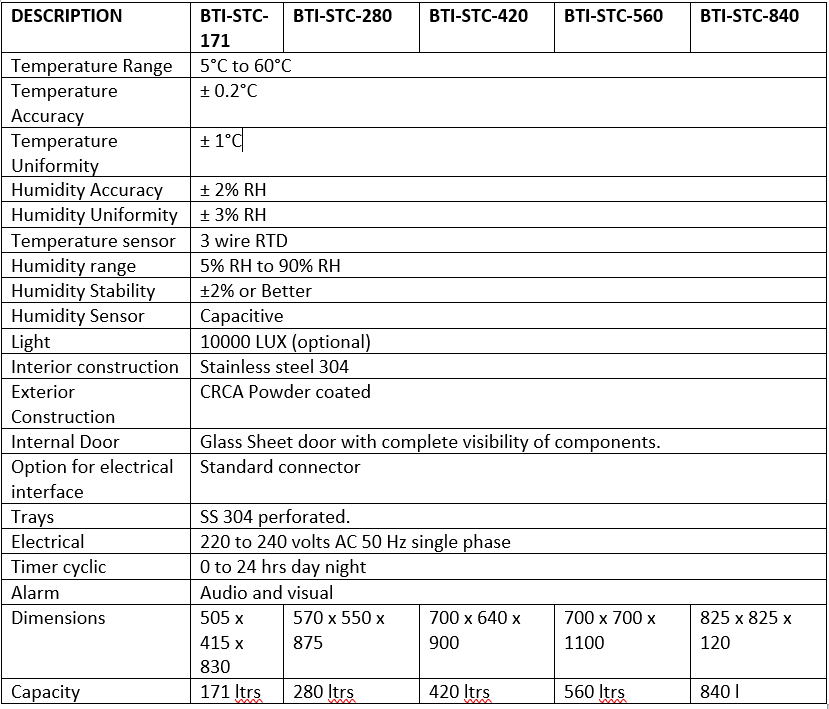
Bio Gene Stability Test Chambers are precision-engineered systems designed to provide controlled temperature and humidity conditions for long-term and accelerated stability testing. Manufactured and exported by Biotechnologies Inc., these chambers meet stringent global standards required for pharmaceuticals, cosmetics, food, packaging, and chemical industries. With uniform air circulation, programmable controls, data logging, and compliance with ICH guidelines (Q1A) and WHO standards, BioGene chambers ensure consistent, reliable performance. Trusted by clients across India and worldwide, BioGene Stability Chambers offer robust construction, energy efficiency, and superior accuracy for both R&D and regulatory testing environments.
BIOGENE Stability Chambers meet Photo stability studies in Pharma, Plastics, composites, Adhesives, Textiles. Cosmetic, Food & Beverages, Personal care Biomedical storage, life science, Electronics etc in compliance with ICH guideline Q1A along with state of art 21 CFR Part 11 software.
Bio-Gene Temperature and Humidity Chambers have: